Research
The ongoing research in the Tunge laboratory is focused on developing mild, waste-free, catalytic methods that facilitate the synthesis of useful small molecules. In addition, they are engaged in detailed mechanistic studies of catalytic processes with the goal of advancing the fundamental knowledge of the chemical sciences. Thus, research projects in the Tunge group span several traditional disciplines with catalysis as the central theme. In particular we are interested in the application of the principles of catalysis toward selective synthesis of biologically active small molecules, combinatorial synthesis, and potential large-scale production of fine chemicals.
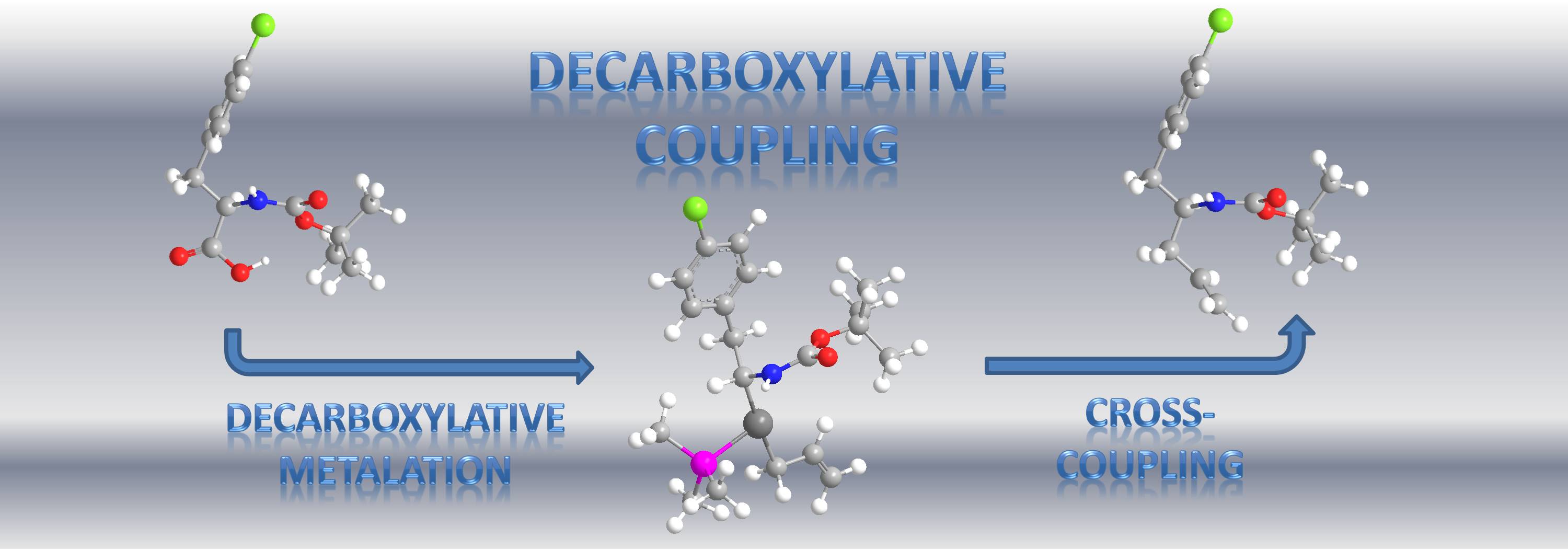
Currently, a major research thrust of the Tunge group is the development of environmentally friendly methods for cross-coupling reactions. While catalytic cross-coupling reactions have made a significant positive impact in the synthesis of chemically-complex small molecules and natural products, such reactions typically require expensive, toxic, or highly basic reagents. Moreover, these reagents produce stoichiometric quantities of hazardous byproducts that are often difficult to remove from the product. With this in mind, the Tunge group is developing catalytic synthetic methods that capitalize on CO2 release as a driving force for the formation of reactive organometallic intermediates. This strategy utilizes ubiquitous carboxylic acids as substrates and avoids the use of expensive, toxic, or highly basic reagents. Ultimately, decarboxylative coupling allows access to a wide variety of useful organic molecules using an environmentally benign method.
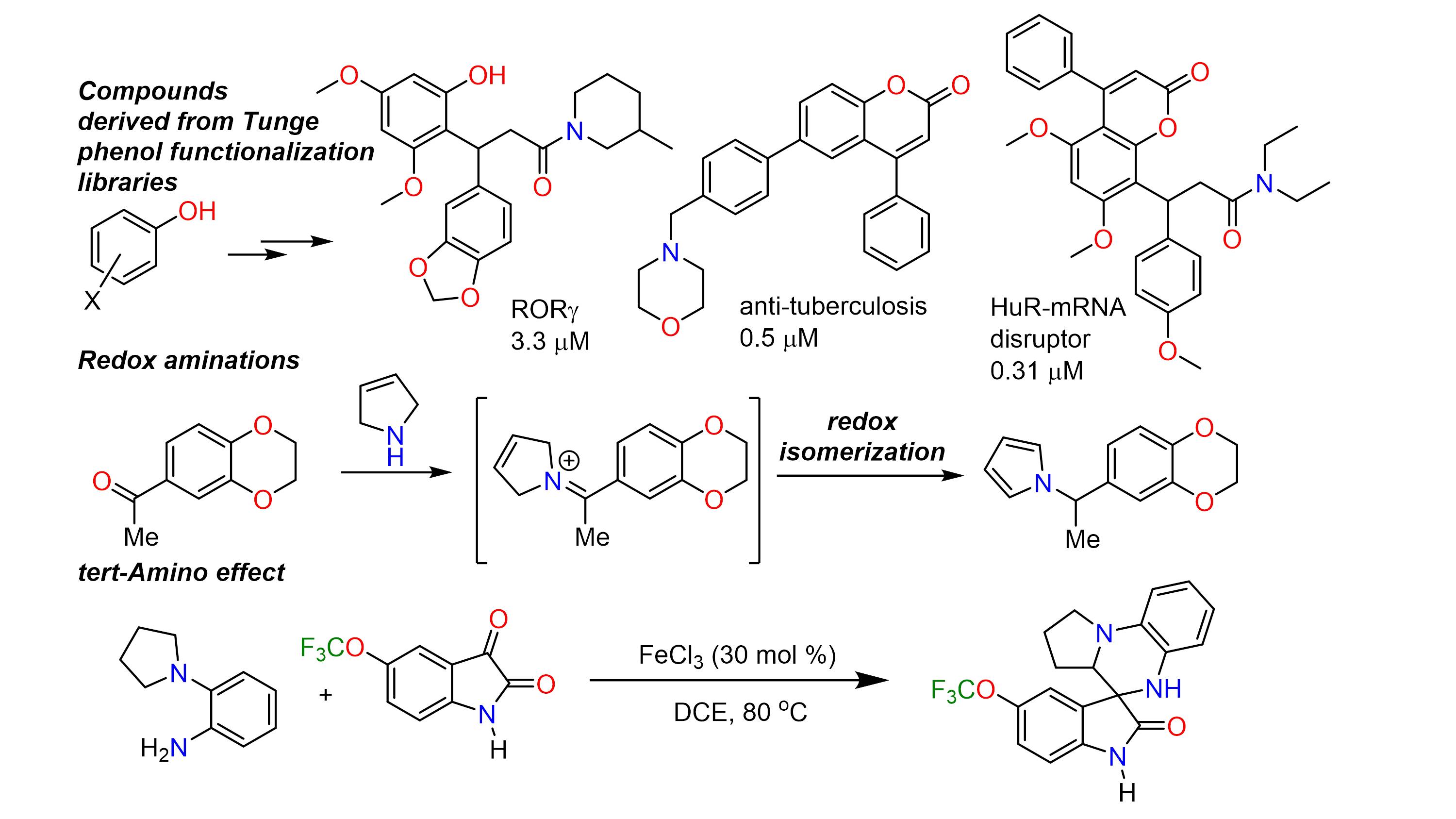
In addition to decarboxylative coupling, the Tunge group is investigating the redox-neutral functionalization C-H bonds to provide products analogous to those produced by well-known cross coupling reactions. Like decarboxylative metalation, such a strategy avoids expensive and/or toxic reagents and minimizes the production of hazardous waste. Since C-H bond functionalization obviates the formation of byproducts, it is ideally suited for parallel synthesis of potentially bioactive compounds for screening. Compounds in our chemical libraries have been shown to inhibit processes implicated in diseases including leishmania, tuberculosis, cancer and hepatitis C.
Lastly, we have a long-standing partnership with Prof. Bala Subramaniam and the KU Center for Environmentally Beneficial Catalysis (CEBC website) to develop homogeneous recyclable catalysts. For example, soluble supported catalysts are effective for the continuous hydroformylation of olefins in environmentally benign CO2-expanded solvents. Moreover, our catalysts are effective for many rhodium-catalyzed coupling reactions including hydroarylations of olefins and aldehydes as well as activation of alkynes toward conjugate addition to unsaturated aldehydes and ketones. Thus, we have developed efficient supported catalysts that can be readily recycled via simple precipitation and filtration or by continuous membrane filtration. Moreover, we have begun to develop similar catalytic approached toward the synthesis of large-scale fine chemicals such as adipic acid and 1,6-hexanediol.